Hormones
- Homeostatic feedback systems
- example: insulin, PTH, cytokines
- insulin: stimulating glucose update into cells
- half-life: 5-6
- SC dosing - flip-flop kinetics: Cmax, Tmax
- PTH
- Teriparatide (N-terminal 34-mer of full-length PTH, approved)
- Anabolic effect - stimulation of osteoblasts
- PTH1R
Cytokine
- HGF - nonlinearity
- Thrombopoietin analogue (PEG-rHuMGDF) - Modeling of thrombopoietic effects
Soluble Receptors
- Etanercept - TNF receptor binding fusion protein
- PASI (Psoriasis Area and Severity Index)
- Sigmoid Emax-model - EC50 2ug/ml
Monoclonal antibodies
- Table 12.2, Table 12.3, Table 12.3 - Approved therapeutic monoclonal antibodies
- Anti-TNF- antibodies
- infliximab (chimeric antibody)
- fully humanized (adalimumab)
- RA biomarker: ACR20 and EULAR improvement
- Therapeutic Antibodies in Oncology
- Rituximab - anti-CD20 -> B cells (ADCC, CDC, apoptosis)
- PK of rituximab - nonlinear in multiple dose
- CLL dose > NHL dose: larger tumor burden and antigen sink
- Bevacizumab: humanized IgG1 monoclonal antibody that binds and inhibits VEGF interaction with its receptor Flt-1 and KDR -> angiogenesis.
- Trastuzumab: targeting HER2 receptor
* The banner is inserted from the article (Hansel et al. 2010).
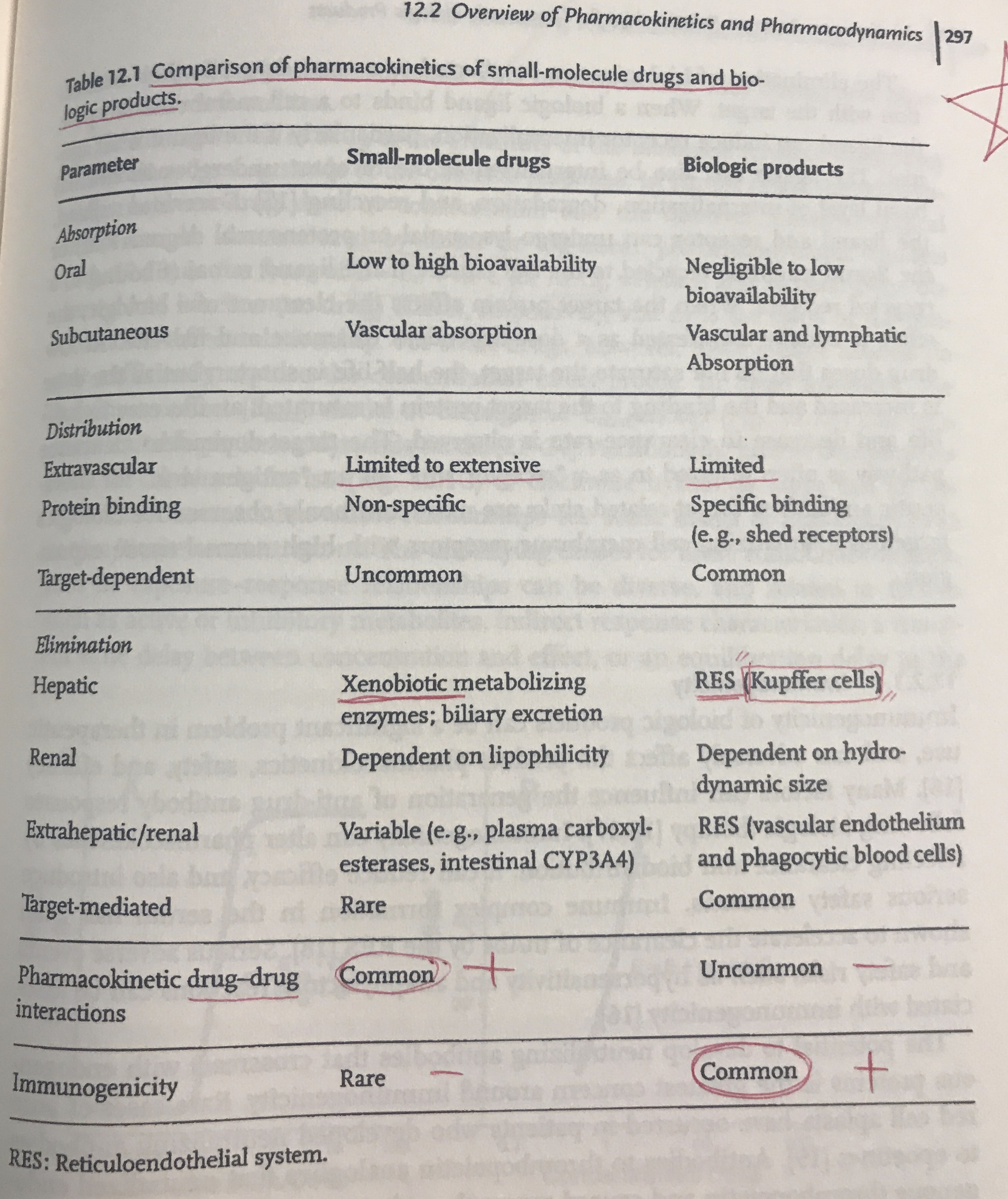
Comparison of PK
Reference
Hansel, Trevor T., Harald Kropshofer, Thomas Singer, Jane A. Mitchell, and Andrew J. T. George. 2010. “The Safety and Side Effects of Monoclonal Antibodies.” Nature Reviews Drug Discovery 9 (4). Springer Nature: 325–38. doi:10.1038/nrd3003.
Meibohm, Bernd. 2006. Pharmacokinetics and Pharmacodynamics of Biotech Drugs : Principles and Case Studies in Drug Development. Weinheim, Germany: Wiley-VCH.